PetroStrategies, Inc. PetroStrategies, Inc. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
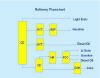
RefiningTopicsRefined Products and QualitiesCrude Oil FractionsCrude oil is processed or refined to produce useable products such as gasoline. The process is very complex and involves both chemical reactions and physical separations. Crude oil is composed of thousands of different molecules. It would be nearly impossible to isolate every molecule and make finished products from each molecule. Chemists and engineers deal with this problem by isolating mixtures of molecules according to the mixture's boiling point range. For example, gasoline molecules might boil in the range from 90 to 400 oF. Home heating oil could be from molecular mixes that boil from 500 to 650 oF. For convenience, the mixtures or fractions are given a name. The following chart illustrates the boiling range and name of the petroleum fraction.
Refined products are produced by combining fractions from the raw crude oil with those from various refinery processing units. These fractions are mixed or blended to satisfy specific properties that are important in allowing the refined product to perform as desired in an engine, for ease in handling and to reduce the undesirable emissions produced when the product is burned. Product Specifications
Gasoline is blended to meet the following specifications:
Jet fuel is blended to meet the following specifications:
Diesel engines are different than gasoline engines, and, as result, have different specifications:
Refinery Operations
Crude Oil DistillationCrude oil distillation is used to separate the hydrocarbons in crude oil into fractions based on their boiling points. The separation is done in a large tower that is operated at atmospheric pressure. The tower contains a number of trays where hydrocarbon gases and liquids interact. The liquids flow down the tower and the gases up. The lighter materials such as butane and naphtha are removed in the upper section of the tower and the heavier materials such as distillate and residual fuel oil are withdrawn from the lower section. Vacuum DistillationThe residua fraction (650 oF. and higher boiling material) from the crude tower can be sent to fuel blending to produce residual fuel oil or No. 6 fuel oil. Often this residua fraction is further separated into a vacuum gas oil and vacuum residua. This unit is operated at a slight vacuum. This allows the hydrocarbons to be separated at lower temperatures and prevent undesirable chemical reactions that would "burn" the material and produce petroleum coke. The vacuum gas oil is sent to the catalytic cracking unit for further processing. The vacuum residua is sent to a coking unit for further processing or to fuel oil blending.
Catalytic ReformingCatalytic reforming is used to improve the quality of naphtha from the crude distillation unit. The catalytic reforming unit uses a catalyst to allow the chemical reactions to take place under "reasonable" temperatures and pressure and "encourage" the desired hydrocarbons to be produced. The motivation for using catalytic reforming can be seen in the following table:
Therefore this process provides higher octane material to the gasoline pool to help meet the octane specifications on the gasoline. The process also produces hydrogen which is used to remove sulfur from refinery streams in the hydrotreating processes. Catalytic Cracking
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Product | PADD I East Coast |
PADD II Midwest |
PADD III Southwest |
PADD IV Rocky Mountains |
PADD V West Coast |
Total |
| LPGs | 3.2 | 3.5 | 5.1 | 1.6 | 2.8 | 4.1 |
| Gasoline | 44.6 | 48.4 | 41.6 | 47.3 | 45.2 | 44.2 |
| Jet Fuel | 5.7 | 6.3 | 9.4 | 4.8 | 17.5 | 9.6 |
| Distillate Fuel Oil | 29.5 | 30.0 | 28.3 | 31.6 | 21.6 | 27.8 |
| Residual Fuel Oil | 7.1 | 1.6 | 4.0 | 2.2 | 5.5 | 4.0 |
| Petroleum Coke | 3.3 | 4.3 | 5.9 | 4.6 | 6.0 | 5.3 |
| Asphalt & Road Oil | 5.1 | 5.3 | 1.2 | 6.1 | 1.4 | 2.7 |
| Petrochemical Feedstock | 1.1 | 1.0 | 3.7 | 0.0 | 0.1 | 2.2 |
| Other | 5.8 | 5.1 | 7.7 | 5.7 | 6.5 | 6.4 |
| Total | 105.4 | 105.5 | 106.9 | 103.9 | 106.6 | 106.3 |
[Source: EIA, Refinery Yield, 2008.]
![]() Please visit the following links for information on refinery utilization, gasoline output and distillate production.
Please visit the following links for information on refinery utilization, gasoline output and distillate production.
![]() Please see Refinery Utilization and Capacity - Monthly and Yearly Data
Please see Refinery Utilization and Capacity - Monthly and Yearly Data
![]() Please see Refinery Capacity Report with data as of January 1, 2013
Please see Refinery Capacity Report with data as of January 1, 2013
![]() Outlook for Refinery Outages and Available Refinery Capacity in the First Half of 2014
Outlook for Refinery Outages and Available Refinery Capacity in the First Half of 2014
![]() Go to the Topic Listing
Go to the Topic Listing
Refinery Economics

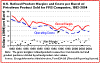 Refinery margins (the difference between raw material costs and product revenues expressed on a per barrel of crude basis) can vary depending on the complexity of the refinery. The more complicated the refinery, the higher the operating costs, but the greater the ability to make higher-valued products like gasoline.
Refinery margins (the difference between raw material costs and product revenues expressed on a per barrel of crude basis) can vary depending on the complexity of the refinery. The more complicated the refinery, the higher the operating costs, but the greater the ability to make higher-valued products like gasoline.
![]() Operating margins for high complexity or cracking refineries (refineries with catalytic cracking units) are found on the Oil & Gas Journal Cracking Spread chart.
Operating margins for high complexity or cracking refineries (refineries with catalytic cracking units) are found on the Oil & Gas Journal Cracking Spread chart.
![]() Gasoline price components are presented in chart Estimated 2011 Gasoline Price Breakdown & Margin Details prepared by the California Energy Commission
Gasoline price components are presented in chart Estimated 2011 Gasoline Price Breakdown & Margin Details prepared by the California Energy Commission
![]() Go to the Topic Listing
Go to the Topic Listing
|
Rising North Dakota oil production and demand spurs two new refineries - March 27, 2013 |
|
|
"Regional refinery trends continue to evolve," This Week in Petroleum - January 7, 2015, Energy Information Administration |
|
|
$430 million Dakota Prairie oil refinery launches operations - Bismarck-based MDU Resources Group and Indianapolis-based Calumet Specialty Products Partners built the $430 million Dakota Prairie Refinery near Dickinson. Officials said Monday that it has begun producing diesel fuel and is expected to start selling it later this month. Crews broke ground on the refinery in March 2013. Once it is fully operational, it will process 20,000 barrels of oil each day into diesel fuel and other products. A barrel is 42 gallons. - PennEnergy - 5/4/15 |
![]() Go to the Topic Listing
Go to the Topic Listing
![]() Check out the following references to learn more about crude oil refining:
Check out the following references to learn more about crude oil refining:
|
, William L. Leffler, Pennwell Publishing. |
|
|
, James H. Gary, Glenn E. Handwerk and Mark J. Kaiser. |
|
|
, Robert A. Meyers. |
|
|
, Robert E. Maples, Pennwell Publishing |
|
|
|
![]() Click on the following links to learn more about refining operations:
Click on the following links to learn more about refining operations:
|
A Quick Lesson in Refinery Economics, Chevron Corporation |
|
|
Refining On-line |
|
What is a Refinery?, Chevron Corporation |
|
|
Refining Primer - SET Laboratories |
![]() Go to the Topic Listing
Go to the Topic Listing
Copyright 2000
PetroStrategies, Inc.
All Rights Reserved